weaken a developer and cause it to underdevelop and
sometimes stain film. Too little agitation during mixing
may cause the powdered chemicals to settle to the
bottom of the mixer and form hard lumps. When these
lumps of chemicals are undissolved and undetected,
they can clog pumps and plumbing during transfer from
the mixer to the storage tank. These lumps can also cause
the solution to be less active.
Agitation mixers circulate solutions through a pump
that causes a stirring action. There are several types of
agitation mixers available. These include large capacity
models for preparing large volumes of solutions and
small models for making small amounts of solution.
Impeller Mixers
Impeller mixers provide thorough, rapid mixing, but
they must be used with care to prevent frothing or
foaming and introducing air into the solution. The
solution must be mixed so a minimum amount of air is
drawn into it. When the shaft is placed in the center of
the container, the impeller causes a whirlpool effect that
introduces excessive amounts of air into the solution.
Furthermore, when the shaft is in the center of a
container, there is very little agitation in the bottom-
center area of the container and undissolved chemicals
pile up directly beneath the end of the shaft (fig. 9-5).
Avoid bumping the shaft or impeller on the sides or
bottom of the mixing vessel. This procedure may bend
the mixer shaft, and a bent shaft produces excessive
vibrations that can ruin the motor bearings.
WEIGHTS AND MEASURES
The different systems of weights and measures used
in chemical mixing and the relationship of the various
units to one another are matters that every photographer
who prepares photographic solutions should under-
stand.
These days, photographic chemicals are pre-
packaged and are usually published in two systems of
weights and measures: avoirdupois and metric. In the
avoirdupois system, chemicals are weighed in ounces
and pounds and are dissolved in pints, quarts, or gallons
of water. In the metric system, they are weighed in
fractions or multiples of grams and are dissolved in
cubic centimeters or liters of water. With a conversion
table, a formula given in one system can be easily
converted to the other.
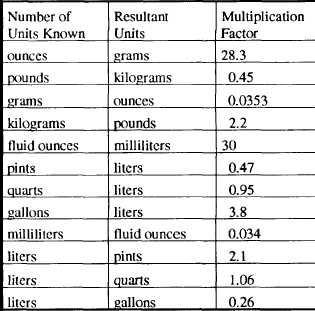
Weight and Volume Conversion
Two systems of temperature measurement are
used: Fahrenheit and Celsius. The Fahrenheit scale
uses °F as a temperature symbol. The Celsius scale
uses °C as its symbol. On the Fahrenheit scale 32
degrees is the freezing point of water, and the boiling
point is 212 degrees. The difference is 180 degrees.
The Celsius scale is 0 to 100 degrees from freezing to
boiling. One degree Fahrenheit is smaller than one
degree Celsius, one Fahrenheit degree being 5/9 of a
Celsius degree. To convert Fahrenheit degrees into
Celsius, subtract 32, multiply by 5 and divide by 9;
that is, (°F – 32) x 5/9 = °C. To convert Celsius to
Fahrenheit, multiply by 9, divide by 5, and add 32; that
is, (°C x 9/5) + 32 = °F.
Some formulas use the word parts as a measure.
They may call for two parts of one chemical, one part
of another, and any number of parts of water. This is
frequently done when two or more stock solutions must
be combined to make the working solution. In such
cases, the word parts means any convenient “volume”
measurement may be used; however, the same measure
should be used for everything required by the formula.
A part may be a fluid ounce or a gallon, depending upon
the total quantity of working solution needed Formulas
use parts only when volume is to be measured.
The term stock solution identifies a concentrated
chemical solution. A working solution is the solution
used for processing. The working solution may be the
same as the stock solution, but more than likely it is a
diluted stock solution.
9-6