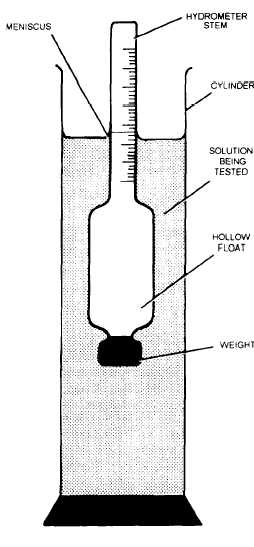
Figure 9-2.–Hydrometer.
reading is taken at the point where the top of the
meniscus intersects the stem of the hydrometer (fig.
9-2).
pH METERS
The acid or alkali state of a solution is measured in
pH values. The pH value of developers and fixers
influences their activity and proper strength. pH is
basically a measure of the degree of acidity or alkalinity
of a solution. It provides an invaluable aid in
determining the degree of accuracy with which the
processing solutions have been prepared. Photographic
developers usually have a pH of 8 to 12, while fixers
range between pH 3.1 and 5.
The following scale indicates the location of acids
and alkalis by their pH value (strength):
pH VALUES
ACIDS
NEUTRAL
ALKALIS
1
2
3
4
5
6
7
8 9 10 11 12 13 14
A pH of 7 is neutral. Working down from this point,
the figures indicate weak acids with a pH of 6 on to
strong acids with a pH of 1. Working up from a pH of 7,
the figures indicate weak alkalis with a pH of 8 to strong
alkalis with a pH of 14.
The pH values are numbered on a logarithmic scale.
From 0 through 6, each number indicates a degree of
acidity 1/10 as strong as the preceding number, but 10
times stronger than the next succeeding or higher
number. A solution with a pH value of 4, for example,
has a degree of acidity 10 times stronger than a solution
with a pH value of 5, but only 1/100 the strength of a
solution having a pH value of 2. When determining the
degree of alkalinity of a solution, figure it in an opposite
manner. From 8 through 14, each number represents a
degree of alkalinity 10 times as strong as the last
preceding number, but 1/10 the strength of the next
higher number; for example, a solution having a pH
value of 11 indicates that the solution has an alkalinity
1,000 times stronger than a solution having a pH value
of 8, but it would be only 1/100 as alkaline as a solution
having a pH value of 13.
Litmus paper is used to indicate whether a solution
is acid, alkaline, or neutral, but it does not indicate the
actual pH value. For this purpose a pH meter should be
used.
A pH meter is an amplifier meter with a scale that
reads from 0 to 14 and an electrode apparatus (Eg. 9-3).
A pH meter has a reference electrode and a pH
measuring electrode, or these two can be combined into
one combination electrode. The pH electrode actually
measures the pH, while the reference electrode that
contains an electrolyte solution is used only to complete
the electrical circuit. The first step in measuring pH is
to establish a point of reference by a standardization
procedure. To standardize the pH meter, you must place
the electrodes in a calibrated buffer solution.
Buffer solutions are available at exact pH values for
this precise standardization. Always select a buffer with
a pH value as close as possible to the pH of the sample
to be tested; for example, use a buffer at a pH of 4.00 to
test a fixer solution or a pH of 10.00 to test a developer
solution. The instrument should be standardized at
regular intervals during a long series of measurements
or before each use.
9-4